Scientific Literature is a permanent record of the collective achievements based on the research, and it is the principle medium for conveying the result of the scientific research. A literature search is systematic, and thorough by nature that pertains to a specific topic. A manufacturer needs to evaluate pre-market clinical investigations to measure the clinical performance and safety of a Medical device. The evaluation is based on the comparison of an equivalent device or the relevant pre clinical studies. Sometimes the clinical investigation data also includes the manufacturer’s own evaluation and report. Literature searching is to identify the data not held by the manufacturer that are needed for the clinical evaluation. The literature search protocols, search reports, and the full text documents of the relevant literatures are used for the preparation of the technical documentation of any Medical Device. meddev clinical evaluation
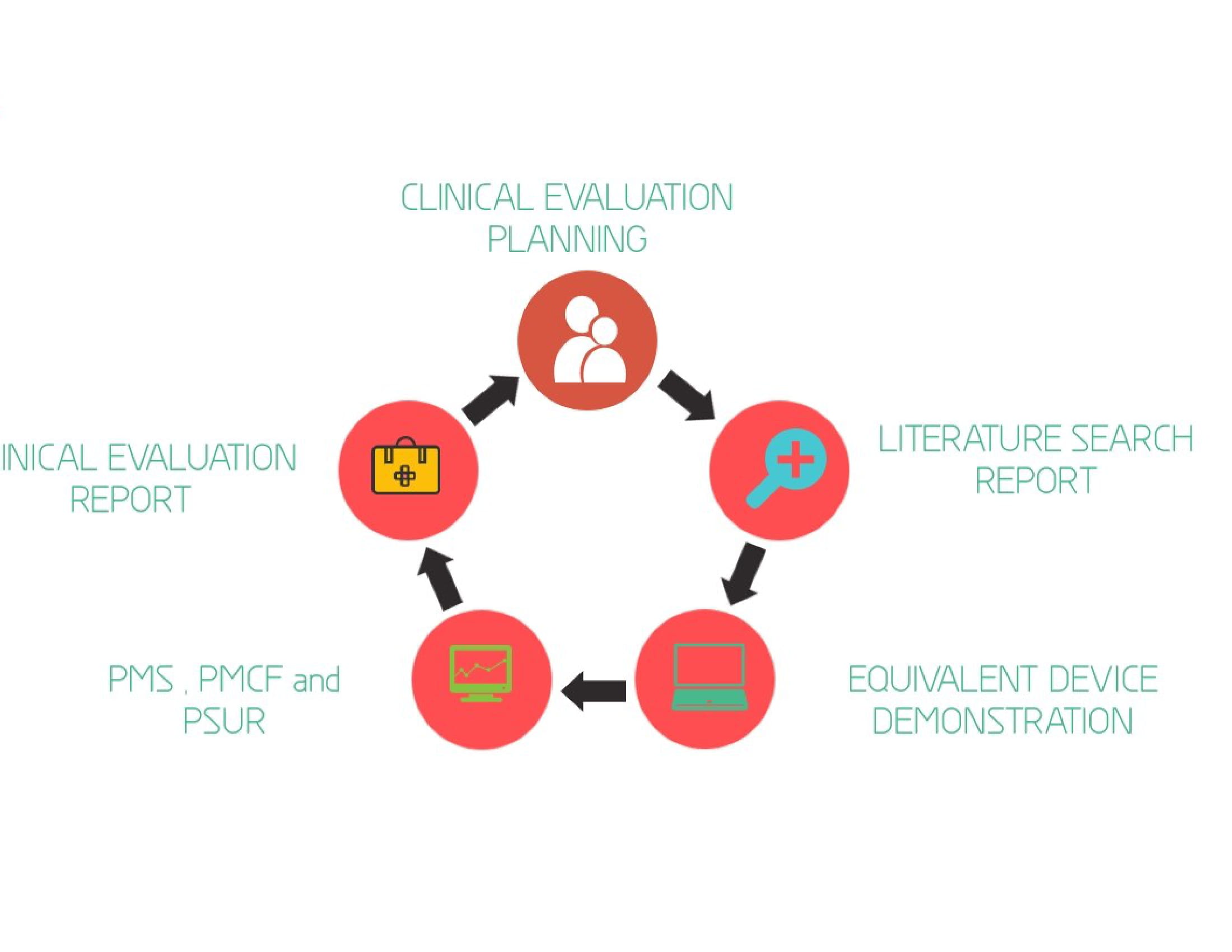
Planning the search strategy is so important to get the clinical data relevant to the device under evaluation and the planning strategy start with the proper understanding of the review questions and the scientific areas covered by the devices such as clinical background, added impurities, clinical hazards and current knowledge/ state-of- the-art. The searching include different scientific literature sources to retrieve the articles which enhances the relevance. The searched articles should be in a way that critically appraises the research in order to answer the formulated questions. Conducting systematic searches results in scientific evidences for the research. For a full systematic review, the search strategy needs to be comprehensive, clear and reproducible. For some Medical devices, a greater part of the clinical evidence is based on the clinical data from Literature searching.
Some Search Frameworks are used to develop the search concepts and terms from the review questions. The input for the review questions are the device description and the intended use of the device. PICO is one of the most available Search Frameworks used in health science to focus on clinical questions. PICO stands for Population/ Patient/ Problem, Intervention, comparison and Outcome. PICOT, PICOS, PICOC are other search frameworks used for developing search terms. A comprehensive search strategy is required, involving multiple literature databases. Some popular scientific literature databases are MEDLINE or PubMed, EMBASE, Web of Science, Cochrane Library and PROSPERO. Additional databases can be used to ensure the adequate coverage of the devices to identify relevant clinical trials. Randomized Controlled trial type literatures are highly prioritized in the selection process. The scientific validity of the information will be more in these type of literatures. Several searches with different search criteria should be applied to obtain necessary and precise data.
Every review report is based on a review question and the searching details such as the name of the database, date of searching, and time frame for the selection of articles are mentioned in the report. The search Keywords are written specifically with the number of hits and the number of relevant articles selected from the list. Different tables are used to maintain search information for the same report according to different scientific databases. Moreover the full text links are listed in one common table and the listing is in accordance with the order of the search string noted in different tables. The links are listed along with the title of the articles. The total number of links listed should be equal to the total number of links selected below the column head “No. of Literatures included After Title/ Abstract Screening”. The redundancy in literatures can be excluded by the person who prepares the report with the justification of duplication with the literature number. We can specify the selection criteria if needed, for example, the device is intended only for particular users or the evaluation is for a particular period. The relevant articles are selected by the abstract screening.